Myelofibrosis Special Report
RUXOLITINIB ON TRIAL
By Zhenya Senyak
The story started long before the American Society of Clinical Oncologists gathered in Chicago.
The beginning
It started four years earlier inTexas and Arizona and 153 different locations where patients, always sick, sometimes dying would swallow a single small pill every day. They were regularly driven from their homes to their local medical center to get pricked and poked, examined and sent back home to hope for the best.
In the whole history of myelofibrosis hope was in very short supply.
And then word leaked out. At first there was only a rumor of success and then the appearance of scientific abstracts, recruitment announcements for new clinical trials. Finally, last month, the story exploded across the Internet .
There is a treatment for myelofibrosis.
Where once there was nothing, now there will be something.
Myelofibrosis
At the acute, devastating end of the myeloproliferative spectrum, MF usually means shortened life expectancy with some intense suffering along the way. As the marrow becomes fibrous and shuts down, blood production moves to new areas. A bloated spleen, thick with new and stored blood, restricts patient movement and appetite, a flood of poorly understood proteins often causes intense itching, bone pain, anemia, night sweats and mind-numbing fatigue.
At advanced stages, those suffering from myelofibrosis begin the process of cachexis, a wasting away syndrome of lost weight, lost muscle mass, and apathy. Eventually, some patients progress to acute myeloid leukemia but the usual cause of death is the crushing effect of myelofibrosis itself.
There is no approved therapy and only limited symptomatic relief.
The only cure for MF is a risky stem cell transplant and that option is only open to the relatively young and healthy.
The rise of hope
There were some signs that hope was on the way. In 2010, the prestigious New England Journal of Medicine published a report on those 153 patients. They had been taking a drug called INCB018424. The study was supported by Incyte Corporation and managed jointly by M.D. Anderson’s Srdan Verstovsek, the report’s lead author and Mayo Clinic’s Ruben Mesa. It was a dose escalation study of yet another JAK inhibitor.
But this time the results of the 14 month study were extraordinary – reduction of spleen volume by an average of over one-third and a 50% overall improvement of myelofibrosis symptom scores.
by an average of over one-third and a 50% overall improvement of myelofibrosis symptom scores.
An open study like this may be promising but it doesn’t prove much to the United States F ood and Drug Administration that approves new drugs based on hard data derived from rigidly controlled clinical trial. The stage was set.
COMFORT
Incyte made the mega-million dollar decision to support two large scale Phase III clinical trials, one in theUnited States, one in Europe. These were the COMFORT (Controlled MyeloFibrosis study with Oral JAK inhibitor Treatment) trials. COMFORT I, the American trial, ended up accruing 309 patients. COMFORT II, inEurope, 219 patients.
Two basic questions would now be answered with scientific clarity. How effective is the drug – now called Ruxolitinib 424 — when matched against a placebo in a blind study? ( In a blind study patients are divided into two groups, each receiving identical looking pills, neither group knowing whether its pill contains the medication or not.) The other question: How effective is the drug compared to the Best Available Therapies, the so-called BAT drugs.
Some quantitative criteria were set up. How much would the spleen shrink, how well would the drug group compare with the placebo or BAT group in reduction of symptoms according to the Myelofibrosis Symptom Assessment Scale. With that, the trial, lasting a little under a year, began.
Back in Chicago
The results formally announced in Chicago at the ASCO conference the first week of June, 2011, were unequivocal. Ruxolitinib was able to stall the rampaging JAK clones, shrink the spleen, and dramatically improve symptoms of myelofibrosis. Incyte filed a New Drug Application with the FDA and asked for fast track approval. It is possible Ruxolitinib will be available for Christmas. But Christmas came early for most patients in COMFORT I and II, many of whom started their trial near death and completed it with a new lease on life..
The Official Results
Both COMFORT trials enrolled high risk MF patients (309 in theUS, 219 inEurope) divided into two arms. In theUS, one arm received Ruxo, 15mg or 20mg depending on platelet levels, the other was essentially untreated, receiving only a placebo.
In Europe, a BAT (Best Available Therapy) drug was given to one arm, Ruxolitinib to the other. The mix of drugs in the BAT arm, including several considered toxic in theUS, was weighted toward commonly used hydroxyurea (47%). Only 4% of the patients received interferons. No Pegasys was included
Despite the design differences, results were generally similar.
Virtually all patients in the COMFORT I Ruxolitinib arm showed some degree of spleen size reduction with 41.9% of patients achieving the targeted end point, a minimal 35% reduction in spleen volume. And nearly half of all patients on Ruxo reported a 50% improvement in symptom burden and quality of life
The results were similar although not so overwhelming in the European version of the trial, reported by Dr. Alessandro Vannucchi of theUniversityofFlorence. While none on the BAT arm of the trial experienced reduction of spleen volume, 28% of patients receiving Ruxo did reach the 35% target reduction. Reduction of severe symptoms and improvement of quality of life was reported by 31.9% of Ruxo participants and none of those on the BAT arm of the trial.
In both COMFORT I and COMFORT II, results were achieved regardless of JAK2 mutation status.
As promising as these results are, the sobering truth is this is the beginning of the battle to eliminate MF, not the end
Robert Rosen, founder of the MPN Research Foundation, considers Ruxolitinib “a huge step forward. It’s no small thing to be the first effective drug for myelofibrosis.” For Rosen, whose MPD Research Association acted as both investor and incubator for scientists working to convert the newly discovered JAK2 mutation into effective drugs for MPN patient, the entry of Ruxolitinib into the FDA approval process is a vital first step. .”We still have a long way to go,” says Rosen,” but the intense level of activity in new drug development, the investigation of new mechanisms of action, and multiple compounds in various stages of the drug development pipeline suggest an unforeseen level of progress.”
` As we go more deeply into this Special Report — and provide supporting data, links and charts to clip and share with our hematologists – the dimensions of this achievement become clearer. Along with celebration, however, there are sobering elements to this astounding success. There are those who didn’t survive, there are failures along the way, side effects to consider and a long road ahead before myelofibrosis can be said to be under control.
What does it mean?
Ruxolitinib can reduce spleen volumes. Everyone on Ruxo got some reduction and some saw complete recovery of normal spleen volumes. Four out of 10 MF patients who took it in the US saw their spleen reduce by more than a third in volume. In Europe, a little better than one out of four patients got those results..
Splenomegaly is not only one result of marrow failure, it is itself 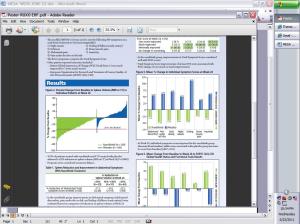 responsible for many life-altering symptoms: inability to eat or breathe normally, exercise, sleep or fulfil other normal daily functions.
responsible for many life-altering symptoms: inability to eat or breathe normally, exercise, sleep or fulfil other normal daily functions.
With Ruxo, nearly half of all patients reported a 50% improvement in symptoms and improvement of quality of life, Then there are those on the placebo or BAT arms taking alternative drugs or none at all. They showed no reduction in spleen size and worsened overall condition. That’s us, MF patients in the pre-Ruxo world.
No free ride
. Generally, JAK2 inhibition is no longer seen as a potential cure for MF but rather one of several approaches that might be taken to attack what is a complex blood cancer. Ruxolitinib, however, did not set out to inhibit the mutant clone but impacted a much broader swath of territory.
If a drug can help you, it can hurt you. Ruxo can help and it carries negative impacts as well. It attacks the JAK2 mutant clone not with a laser guided missile but a shock and awe carpet bombing of the JAK 1 and 2 kinases and the JAK-STAT pathway. Impacting this cellular signaling link – see the sidebar on the JAK STAT pathway – suppresses the marrow, worsens anemia, lowers platelet counts and may well have other adverse effects. For those with MF already suffering from compromised marrow, anemia and thrombocytopenia, this is bad news. The study did show blood counts rebounding and hemoglobin rising to former levels after a few months on the trial. Lowering of platelets continues to be a problem since those with MF and very low platelet counts are not candidates for Ruxo. Verstovsek is confident many response issues can be safely treated at a clinical level. “We can manage these issues and balance toxicity with efficacy,” he told MPNforum, “by calibrating dosage.”
Why invest big bucks in MF anyhow?
In the carefully calibrated dance between Business and Science, patients with myeloproliferative neoplasms stand as poor relations, spectators at a festival honoring major diseases and blockbuster drugs. And among the MPNs, those who are last in line, least numerous and most in need, are those with high risk advanced myelofibrosis.
Clinical trials are expensive. We were told the numbers aren’t made public – “We haven’t given this sort of guidance in the past…” Drug companies are not compelled to tell us how they arrived at the enormous sums claimed necessary to bring a new drug to market.
An Incyte spokesperson did quote a report that might be illuminating: “Finding new cures is an extremely expensive and risky proposition…Estimates about the costs of developing a new drug vary widely from about $800 million to nearly $2 billion per drug. …Recently Pfizer announced that it is investing $800 million just for a set of Phase III trials for a single drug.”
Seriously, who is going to spend $800 million to serve a patient population of a few thousand in the US when insurance might not cover the prescription anyhow?
As MF patients, hat in hand, we can be grateful for any product promising symptomatic relief. But, considering our small numbers and the huge required investment, it is reasonable to wonder at the corporate strategy that would put so much capital in play when there are competing products in the pipeline, and several promising genetic therapies being explored.
Paul Friedman, president and CEO of Incyte, is up front about it. “There was a list and MF was on the list. It seemed the fastest road to market since there was no available therapy for a serious disease. ” Another disease on that list has much greater market potential. It turns out that the JAK STAT pathway is significant to treatment of rheumatoid arthritis, one of the major diseases afflicting ever aging populations. (And that same pathway is involved in gout and psoriasis, as well. where an offshoot of INCB18424 is being marketed as a cream “with spectacular results.”)
While there might be 18,000 MF patients in the United States– and only a subset of those would be advanced enough to qualify for Ruxo therapy — there are over 1.5 million people with rheumatoid arthritis and another 5 million with such related rheumatoid diseases as fibromyalgia and gout in the States. The worldwide market is orders of magnitude larger.
So it’s not about us. Corporate strategists might be sympathetic to the plight of victims of advanced MF but they’re also after much bigger fish and we’re just swimming in the same pond. In a very strange way, we just lucked out.
To have a shot at the RA market, Incyte needed capital, partners, product development, and recognition. In the course of proving its bio-engineering and drug marketing capabilities, Ruxolitinib helped bring all that about.
For some, it is all about us.
For front line hematologists, scientists in clinical practice who specialize in the MPNs, and those who further devote their lives to research and the care of myelofibrosis patients, we are the bottom line.
And they are both relieved and excited about Ruxolitinib.
“For the first time we have a treatment (for MF),” said trial investigator Dr. Claire Harrison of Guy’s and St. Thomas, London “that meaningfully reduces spleen size and addresses the burden of disabling symptoms.”
“It’s an extraordinarily successful study, “said Srdan Verstovsek, “in terms of reducing symptoms…shrinking the spleen. We have things to work on, combination studies to block the pathway, combine maybe with Pegasys, thalidomide or lenalidomide. We’re working on quite a few. And we have to do a study on patients with low platelets, find a way to get them this therapy. I think we have to accept the drug is eliminating symptoms not curing MF. But given such a degree of suffering and debility, the drug is valuable.”
Verstovsek also sees Ruxo as useful in preparing MF patients for bone marrow transplant. “Often when patients arrive for BMT they are in such terrible condition that the odds are against them. We can improve that, improve their nutrition and help them get more fit before starting that process.”
Will Ruxo succeed as a frontline therapy for MF and other MPNs?
Clinical trial results and efficacy in the field are necessary but not sufficient to assure Ruxolitinib of success. The jury will remain out on that one beyond the anticipated December FDA approval date. There are unknown marketing and scientific pitfalls ahead, long-term studies of survival and durability of response that will building on on-going data from the trials. And then there’s the issue of acceptance of the drug in clinical practice and insurance reimbursements. Finally, although Ruxo has a good headstart, there’s real competition just over the horizon.
A final note
As MPN/MF patients we don’t have many options. We can only wait to see how this part of the amazing Ruxolitinib story plays out. The men and women who worked on the development and testing of this drug already have an achievement beyond the balance sheet. The history of myeloproliferative disease will credit them with creating not an oral JAK inhibitor drug but the first effective therapy for advanced myelofibrosis.
With any luck at all, we too will be part of that history.
___________________________
© Zhenya Senyak and MPNforum.com, 2011. Unauthorized use and/or duplication of this material without express and written permission from this blog’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Zhenya Senyak and MPNforum.com with appropriate and specific direction to the original content.


Comments on: "Ruxolitinib" (14)
I was diagnosed in 2005 w/primary myelofibrosis. In march 2012 I was put on JAKAFI. This med has helped w/blood counts and reduced spleen size a little.I still have bone pain fatigue,night sweats though not as frequent but lately having chest discomfort arm pain back pain dizziness and frequent severe head aches.Anyone else have any of this?
I think It will be quite interesting and perhaps important for MPNforum to see how individual panelists answer Cheryl. Whether acting as her practicing personal physician or consultant with specific clinical advice, Rx, tests etc. for her medical problem(s) or will they answer her question with expert current understandings and concepts, but in broad general terms, ending as we do with advising her to see an expert privately.
True enough Arch BUT the roundtable Doctors won’t even see the question unless Cheryl has sent it to MPN Clinic via mpnclinic@gmail.com. That’s the only route to reach Mary, the Project Coordinator, who will log it in and start the process.
This will be a good question to pose to the clinic. I had a terrible time with radiation this time . I was just able to finish 5 treatments and my WBC and rbc counts went to zero and my platelets to 35. I had several transfusions …then to surgery with 2 tears in my retina. Overall, the radiation did not help amd my spleen is huge. I take HU 3 days a week, but nothing else. But, I am alive and hoping for better days.
Good idea, Cheryl…Sorry about your hard time with radiation and difficulties with symptoms. How about framing a question for the MPN Clinic doctors and sending it to mpnclinic@gmail.com. The first MPN Clinic Report will be published September 15 but as responses arrive we get them out to patients Immediately. Good luck
[…] presented findings that differed little from those presented at the ASCO meeting five months ago https://mpnforum.com/2011/10/12/ruxolitini/ , But they are like rock stars on tour and you don’t fault the Rolling Stones for doing […]
Barbara Beckman was courageous in going through the early Ruxolitinib trials and courageous in telling her story here https://mpnforum.com/2011/10/12/on-trial-for-her-life-2/
She’s one of the quiet heroes this issue has been about. Thank you, Barbara…
Since we vary in our DNA, we are going to react differently to different medications. Ruxolitinib is not going to help everyone. That is the physician’s dilemma. This may not be the correct medication for you.
We have heard before that discontinuing Ruxolitinib is difficult. Patients have told us how hard it is. I don’t know if they quit all at once, or gradually. I know Dr. V. advocates a gradual withdrawal.
As for me, the 3 1/2 years I have been on the trial have made me feel much better. Maybe it will keep on working for me, or maybe it will not. It is such a new thing that we are still learning. Until then…………….
Barbara Beckman, MF
A picture of the JAK-STAT pathway and its dysregulation in MF was part of the original Ruxolitinib story. You should be able to find it here: https://mpnforum.com/jak-stat-pathway-follow-the-yellow-brick-road/
I’m also JAK2 Neg., like nearly 50% of us with MF… I can understand the confusionm since Ruxo is described as a JAK2 inhibitor. The article on Ruxolitinib — you can find it in the search box — refers to the equally positive response JAKneg MF patients had foreshadows positive negative effects: : “Generally, JAK2 inhibition is no longer seen as a potential cure for MF but rather one of several approaches that might be taken to attack what is a complex blood cancer. Ruxolitinib, however, did not set out to inhibit the mutant clone but impacted a much broader swath of territory.
If a drug can help you, it can hurt you. Ruxo can help and it carries negative impacts as well. It attacks the JAK2 mutant clone not with a laser guided missile but a shock and awe carpet bombing of the JAK 1 and 2 kinases and the JAK-STAT pathway. Impacting this cellular signaling link – see the sidebar on the JAK STAT pathway – suppresses the marrow, worsens anemia, lowers platelet counts and may well have other adverse effects. For those with MF already suffering from compromised marrow, anemia and thrombocytopenia, this is bad news.”
Hi, Cheryl… it doesn’t make much sense to me. Ruxolitinib is a JAK1/JAK1 inhibitor, specifically designed to reduce the impact of the mutant JAK clone so I would think being JAK2+ would increase the likelihood the drug would help reduce splenomegaly and improve quality of life. The findings by Tefferi & his Mayo associates sheds new light on long-term effects and is part of the discovery process that is likely to go on for years. If you do a search on this site for Ruxolitinib you should find a wealth of data you can show your hem…or a new one.
I think I am confused. I am JAK-, not positive. I do not really understand what that means other than my hem says the drug will do me no good.
I have been so encouraged by reading the results of this medicine. But, my hematologist told me last week that since I am JAK2-, the drug would do me no good. I was stunned. So, I guess it is back to Hydrea & radiation for me. Any thoughts?
Hi Cheryl,
I believe you Hem. is wrong. If you have an enlarged spleen, chances are that it is certainly worth a try. I have read many accounts where Ruxo 424 has helped MF sufferers with both JAK+ and Negative.
I am PPV-MF JAK2+ and could well end up the “poster boy” for this drug. Spleen reduction from 25 cm to 13 cm in first month! Now they have to search for it.
I missed out on the trials, but am being supplied on a compassionate basis.(Ruxo is not yet approved in Australia)
I have been on 424 for one year and have more energy now than I have had in 10 years and most of my blood is coming back in normal parameters. No effect to platelets and Hb115.
To coin a phrase, I can live with that!